Krystal Biotech Reports Positive News from Phase 2 Clinical Trial of KB103
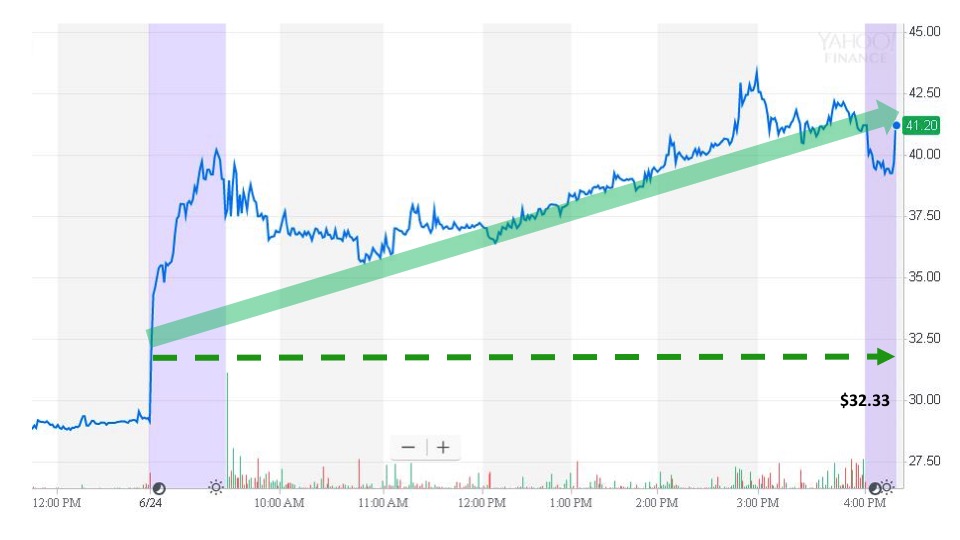
On June 24, Krystal Biotech (KRYS) reported positive news from its Phase 2 clinical trial of KB103. The treatment was effective and well tolerated by patients suffering from recessive dystrophic epidermolysis bullosa (RDEB). In addition, the FDA awarded Regenerative Medicine Advanced Therapy (RMAT) status to Krystal Biotech for KB103. Krystal Biotech is a gene therapy company dedicated to developing novel treatments for patients with rare dermatological diseases.
Sonal issued an alert to subscribers at 8:00 am. The next trade took place at 8:24 am for $32.33. Regular market trading opened for the day at $37.83. The stock continued to move higher throughout the day before closing at $41.20. That makes an event-day gain of 27.4%.
If you want to learn more about trading on clinical trial results, visit the Knowledge Center.
To see the latest weekly webinar, you can visit our Live Webinar page.
Subscribe here if you would like to start receiving these signals in real-time and start trading!